Integrated Developmental Design Laboratory
Research Overview
The field of biomaterials and advancement in microfluidic technologies have permitted the study of fundamental cellular processes in physiologically relevant microenvironments. The marriage of these tools enables the controlled and dynamic administration of biophysical and chemical cues necessary to drive stem cell differentiation and maturation processes. Given the strength of these platforms, our lab focuses on bridging the gap between fundamental stem cell biology and clinical application of stem cell derivatives.
Our Lab is Generously Supported By
Embryonic Development
Insight into human development is limited by the lack of controlled model systems that can recapitulate the complex signaling pathways that drive organism formation. While directed differentiation approaches have begun to unveil the mechanistic underpinnings of how a single stem cell can undergo fate specification, we still lack complete insight into how our organs' elaborate architecture and patterns arise. Gastrulation marks a nascent developmental milestone, requiring coordinated migration and division events to specify the three germ layers that give rise to all tissues within our body.
Using a combination of human-induced pluripotent stem cells, computational image processing tools, and micropatterning technologies, we are able to re-create aspects of self-organization during human development (Smith et al. PNAS). Our current efforts involve studying the roles of oxygen tension, mechanics, and cell-cell interactions during these processes, in order to create spatially restricted higher-order tissues such as the liver.
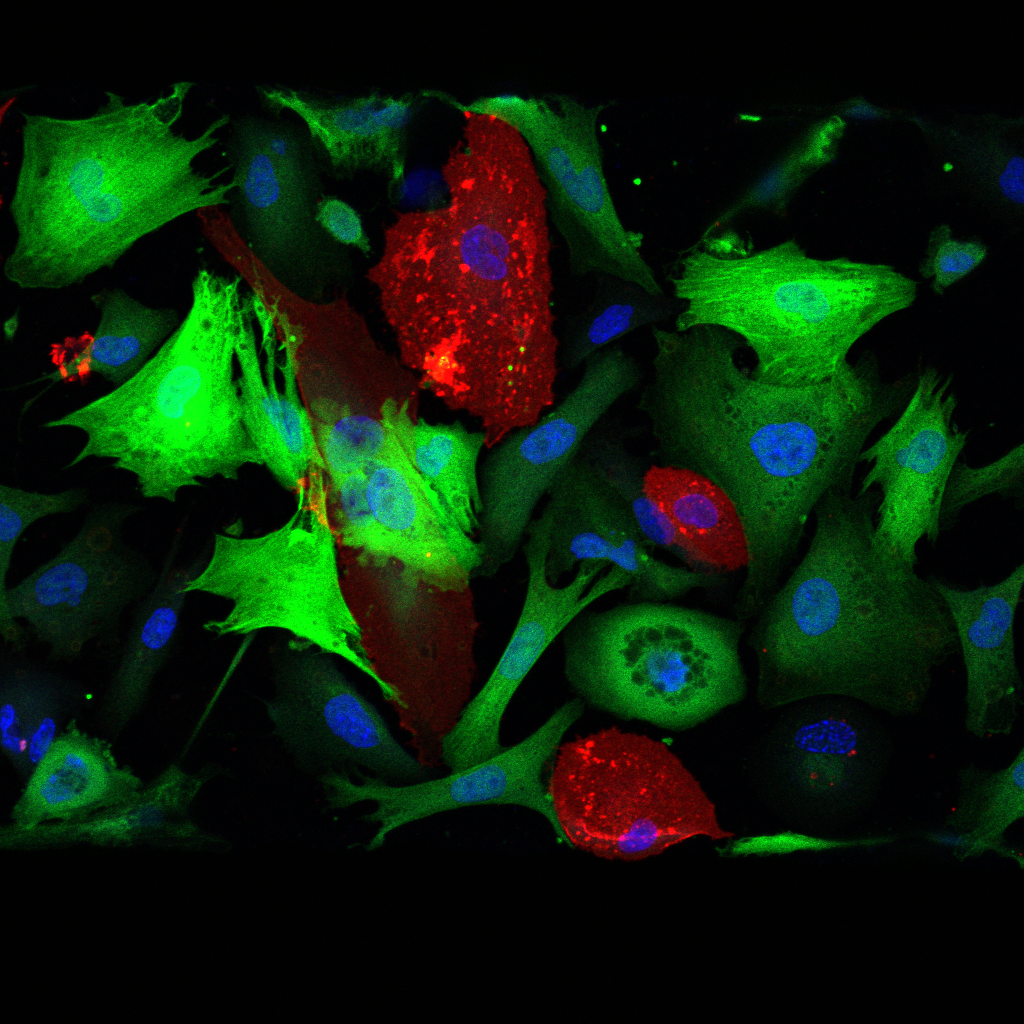
Liver Differentiation and Morphogenesis
Morphogenic events driving liver development involve Notch-mediated hepatoblast specification, a binary cell fate decision that gives rise to either hepatocytes or cholangiocytes. Here, juxtracrine signal transduction is activated between neighboring cells that express Notch ligand and receptor pairs, leading to the formation of biliary ducts or hepatocyte plates. While these cell-cell interactions are key to stem cell differentiation, mechanical cues imposed by extracellular matrix rigidity can tune progenitor stemness (Smith et al. Science Advances) and inhibit Notch signaling.
Our goal is to manufacture engineered hydrogel systems that elicit architectural and physical cues that mimic the nascent liver microenvironment, by leveraging synthetic biomaterial scaffolds and 3D printing technologies. By directing hepatic specification in these micro-physiological systems, we aim to further understand the crosstalk between cell-cell and cell-matrix interactions during development and increase the functionality of stem cell-derived hepatocytes and cholangiocytes for downstream application in regenerative medicine settings.
Microfluidic Organoid Models
Organoids, which can be derived from adult progenitor or pluripotent stem cells, have transformed traditional in vitro model systems. These self-renewing, multicellular ensembles, not only mimic aspects of parenchymal tissue structure, composition, and function when cultured in three-dimensional settings, but also enable disease modeling, drug discovery, and tissue regeneration. As organoids expand in size, diffusional limitations of oxygen, nutrients, and waste exchange lead to necrosis, a phenotype that can be ameliorated with the integration of a functional vascular bed. Our lab seeks to leverage microfluidic technology to integrate stem cell-derived vasculature (Smith et al. Cell Reports) with hepatic organoids, to study the roles of fluid shear stress and paracrine signaling in the context of liver development and regeneration.
















